End 2019 Final Stage of designations in NANDO - 9 NBs designations were published for MDR and 3 NBs for IVDR
Notified Bodies are responsible for assessing medical devices (MDs) and diagnostics (IVDs). They are an indispensable part of the regulatory system since they grant a CE mark to each device before it can be placed in the EU market. Both new regulations, MDR 2017/745 and IVDR 2017/746, haved introduced new obligations for Notified Bodies and require the assessment of more products than ever before.
At the end of 2019, Notified Bodies designations published by NANDO for MDR and IVDR legislations were (in chronological order) :
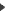 MDR
MDR
BSI UK - 0086 - United Kingdom,
TÜV Süd - 0123 - Germany,
DEKRA - 0124 - Germany,
IMQ - 0051 - Italy,
TÜV Rheinland - 0197 - Germany,
Dare!! Services - 1912 - Netherlands,
BSI Group The Netherlands - 2797 - Netherlands,
DEKRA Certification B.V. - 0344 - Netherlands,
MEDCERT - 0482 - Germany
(3 are given to be awaiting publication still today, January 21, 2020).
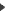 IVDR
IVDR
DEKRA - 0124 - Germany
BSI UK - 0086 - United Kingdom,
BSI Group The Netherlands - 2797 - Netherlands
 Exhaustive Accreditation detailed lists are available from the EU Commission website at :
Exhaustive Accreditation detailed lists are available from the EU Commission website at :
https://ec.europa.eu/growth/tools-d... for the MDR designated NBs.
https://ec.europa.eu/growth/tools-d... for NBs designated for the IVDR.